blood drop test copper sulfate iron|copper sulfate test tube : manufacturers Add a drop of blood to the Copper Sulfate solution from a height of about 1 cm. Observe the behavior of the drop. Change the Copper Sulfate solution after every 25 tests, daily, and if the . WEBAn unapologetically candid drama that looks at the modern workplace through the lens of the people who help wake America up, pulling back the curtain on early morning TV.
{plog:ftitle_list}
Resultado da Joined January 2023. 49 Following. 89.5K Followers. Brenda Trindade @bre_trindade. 1 day ago. meu jeitinho de pedir por anal 🌚 . Brenda Trindade @bre_trindade. 5 months ago. calcinhas me incomodam muito, prefiro ficar sem 🫣 .
One of the standard methods involves placing a drop of blood into a test tube of copper sulfate solution: if the drop of blood sinks to the bottom in an acceptable amount of time, the donor qualifies. If the drop of blood floats or takes too long to sink the donor is deferred.
A small blood sample obtained through a finger prick test--also know as a . The study showed that a significant number of the prospective blood donors deferred for having low hemoglobin by the copper sulphate method turned out to have anemia . A small blood sample obtained through a finger prick test--also know as a hemoglobin test or Hgb--is all that is required to determine base iron levels. This test is always .Add a drop of blood to the Copper Sulfate solution from a height of about 1 cm. Observe the behavior of the drop. Change the Copper Sulfate solution after every 25 tests, daily, and if the .
To safeguard donors, blood services measure haemoglobin concentration in advance of each donation. NHS Blood and Transplant's (NHSBT) customary method have been capillary . Specific gravity of 1.053 corresponds to an Hb level of 12.5 g/dL. A drop of blood, allowed to fall into a copper sulfate solution of specific gravity 1.053, becomes encased in a sac of copper proteinate, which prevents .
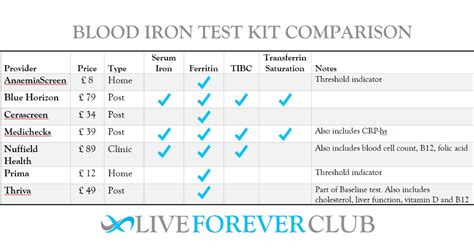
nhs blood test for iron
The copper sulphate (CuSO4) method is mostly used to ensure a certain Hb level for blood donation. A blood droplet is allowed to fall into copper sulphate solution of a specific gravity, equivalent to that of blood with the cut . Blood donor hemoglobin (Hb) estimation is an important donation test that is performed prior to blood donation. It serves the dual purpose of protecting the donors’ health against anemia. Hemoglobin screening is one of the most important tests performed during a donor’s interview process to determine an individual’s eligibility to donate blood products. Donation facilities frequently use the fingerstick method to .
Add a drop of blood to the Copper Sulfate solution from a height of about 1 cm. 3. Observe the behavior of the drop. 4. Change the Copper Sulfate solution after every 25 tests, daily, and if the solution turns cloudy during testing. . specific gravity test method, a Copper Sulfate Solution with a correct specific gravity of 1.0531 at 25°C . The use of the copper sulphate method is based on the idea that when a drop of whole blood is allowed to fall into a solution of copper sulphate, an insoluble copper proteinate is created. An iron blood test can show whether you have too much or too little of this important mineral in your blood. Find out why your doctor might call for this test, and what the results mean.Reactions of iron with complex substances. At a temperature of 700 ᵒC (1292 ᵒF), iron reacts with benzol with the formation of iron carbide:. 18Fe + C₆H₆ = 6Fe₃C + 3H₂. At room temperature in air and in the presence of moisture, iron corrodes (corrosion is the spontaneous disintegration of metal under the impact of the en .
Ferritin is not the same as iron. Ferritin is a protein complex that stores and releases iron to maintain the body's iron levels. Iron, on the other hand, is a mineral essential for a variety of bodily functions, including the production of hemoglobin — the molecule in red blood cells that transports oxygen in the blood. Iron also plays a crucial role in other important .
1. There is a brown coating on the iron nail which was dipped in the copper sulphate solution, whereas the iron nail placed in petri dish shows greyish colour of iron. 2. The colour of the copper sulphate solution in which the iron nail was dipped turns light greenish, whereas the solution of copper sulphate in the other test tube does not .Find step-by-step Physics solutions and the answer to the textbook question A higher level of hemoglobin in the blood increases the blood's density. This is the basis for a simple test that can be used to see if a prospective blood donor has a high enough hemoglobin level to donate safely. A drop of blood is placed in a copper sulfate solution, and the time for the drop to sink to the .This standard operating procedure describes the preparation of copper sulfate (CuSO4) solution for use in pre-donation hemoglobin testing. CuSO4 solution with a specific gravity of 1.053 is used to estimate if a donor's hemoglobin is above or below 12.5 g/dL. The SOP outlines weighing copper sulfate crystals, making a stock solution, diluting it to the proper concentration, quality . When an iron nail is dipped in copper sulphate solution, a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solutio.
Early qualitative methods for assessing Hb in clinical settings include the copper sulfate technique . Cost/test, USD (2018) Copper sulfate technique (CST)a: . 80, 100, 120, and 140 g/L) that are mounted onto strips. A drop of blood is placed onto a moveable piece of filter and compared to the shades of red on the color scale. ∼0/box .The customary approach of National Health Service Blood and Transplant (NHSBT, the national blood service of England) has been a gravimetric method (copper sulphate test) carried out on finger‐prick capillary blood taken immediately before donation, followed by a spectrophotometric test (HemoCue) with venous blood for those who fail the .
colored Prussian blue precipitate or of the deep blood-red complex, the confirming test for iron(III). Concepts • Complex ions • Oxidation–reduction • Oxidation numbers • Transition metals Materials Iron(III) chloride solution, 0.02 M, FeCl 3 6H 2 O, 60 mL Colorful Iron Complexes Worksheet Iron(II) sulfate solution, 0.02 M, FeSO 4 7H 2Total Copper (Blood) Does this test have other names? Total copper serum test . What is this test? This test measures the total amount of copper in your blood. Normally most of the copper in your blood is carried by a protein called ceruloplasmin. Adults have 50 to 120 milligrams (mg) of copper in their body, mostly in muscle and the liver.Iron nails, Copper Sulphate solution, Test Tube, Clamp Stand, Sandpaper. Theory. The colour of pure iron is greyish. Pure copper is a reddish-brown metal. The presence of Cu2+ ions causes the aqueous C solution of copper sulphate to be blue. The presence of Fe2+ ions causes the aqueous solution of ferrous sulphate to be pale green. Copper Sulfate. Like the Ferroxyl Test, testing with copper sulfate (CuSO4) provides a readily visible indication of the presence of free iron on a surface. The copper sulfate is a dilute solution of water, copper, and .
The copper sulfate (CuSO 4) specific gravity method is the traditional method being used for donor screening at many blood . Each drop of blood added to the solution affects the specific gravity, . We have provided data that the CuSO 4 method still stands the test of time and this method can be retained as the primary screening method .
CBC (red blood cells): Healthy results for a red blood count are 5 to 6 million cells per microliter (cells/mcL) for men and 4 to 5 million cells/mcL for women. Values below these levels may . A drop of blood, allowed to fall into a copper sulfate solution of specific gravity 1.053, becomes encased in a sac of copper proteinate, which prevents dispersion of fluid for 15 seconds. If the specific gravity of blood is higher than the solution, the drop will sink or else it will remain suspended for some time.
Edit to clarify: In my years of blood donation the test for iron level was to put a drop of my blood in a solution of copper sulfate and time how long it took for the drop to fall to the bottom. As I got towards the end of my donation career I began to fail this test however a more accurate test would then be done and I would pass that test. A drop of b lood, allo wed to fall into a copper sulfate solution of specific gravity 1.053, becomes encased in a sac of copper proteinate, which prevents dispersion of fluid for
The copper sulphate (CuSO4) method is mostly used to ensure a certain Hb level for blood donation. A blood droplet is allowed to fall into copper sulphate solution of a specific gravity, equivalent to that of blood with the cut-off hemoglobin level, e.g. 12.5 g/dL (125 g/L). If the drop of blood floats or takes too long to sink, the donor is .
An excess of copper (II) sulfate solution (to make sure that all the iron is reacted) will be added to a known amount of iron. The metallic copper produced will be weighed. These weighings will be used to calculate the moles of iron used and the moles of copper formed. If equation (1) is correct, the moles of copper should equal the moles of iron.
Fig. 1.1 : (a) Iron nail dipped in copper sulphate solution; and (b) Iron nails and copper sulphate solutions are compared Test tube Copper sulphate solution (before experiment) Test tube stand Reaction mixture (after experimnet) 6. Compare the intensity of blue colour of copper sulphate solution before and after the experiment in tubes A and B . Ferrous sulfate is an iron salt that is commonly used as an iron supplement. . Hemoglobin allows red blood cells to transport oxygen around the body as well as giving red blood cells their color. Only the iron component of the iron salt is utilized; therefore, iron supplements usually state the amount of the iron salt (for example, ferrous .In the reaction between an iron nail and copper sulphate solution, metallic iron is converted into ferrous ion (Fe 2+), and cupric ion (Cu 2+) is converted into metallic copper. Fe(s) + CuSO 4 (aq) ? FeSO 4 (aq) + Cu(s) Experiment. To perform this experiment, apparatus and materials are required: two test tubes, two iron nails, a test tube .During the donor screening process, the potential donor's blood drop sinks to the bottom of the copper sulfate solution within 15 seconds. The interpretation of this test. The donor has an acceptable hemoglobin and hematocrit to donate
More specifically, a TIBC test shows the amount of transferrin in your blood. Transferrin is a protein your liver makes that regulates the absorption of iron into your blood.. Your body needs iron to make healthy red blood cells.Red blood cells carry oxygen from your lungs to the rest of your body.

copper sulphate test nhs
WEBResultados. Por favor, verifique o usuário e a senha em seu protocolo de atendimento ou entre em contato conosco por telefone para receber orientações.
blood drop test copper sulfate iron|copper sulfate test tube